- 2012年度
- 2011年度
- 新たに判明 がんの転移を促進するメカニズム
(2012/03/23) - 神経発達と加齢における5-hmCを介したエピジェネティクス
(2012/03/23) - TACEを調節するiRhom2は、リステリア菌やLPSの反応により産生されるTNFを制御している
(2012/03/09) - HIV-2って何?
(2012/03/09) - 自殺遺伝子を持ったiPS細胞
(2012/03/09) - リハビリって神経幹細胞も殖やすんです!
(2012/02/24) - 癌幹細胞を制御するHippo pathway
(2012/02/10) - 吸血鬼が若い血を好むのには根拠があった?!~若い生き血でボケ防止~
(2012/02/10) - 癌幹細胞を特異的に標的とした治療法を開発できる可能性!?
(2012/01/27) - 骨の再生には、本来体を守る役割を持つはずのサイトカインは邪魔になる!?
(2012/01/27) - 骨格筋の老化は防げる?
(2012/01/13) - 意外に他力本願???他者の掘ったトンネルを行く癌細胞
(2012/01/13) - 新しいRNA間コミュニケーションのカタチ@筋肉
(2011/12/23) - 精子形成に必須なタンパク質Miwiによるトランスポゾンの発現抑制
(2011/12/23) - Dying well with dementia
(2011/12/09) - Recent insights into the epigenetic regulation of the hair follicle bulge stem cells
(2011/12/09) - ヒトiPS細胞から誘導した神経幹細胞における脳梗塞に対する移植治療の可能性
(2011/11/25) - 体細胞の再プログラム化を阻む"小さなRNA: miR-34"
(2011/11/25) - 薬剤性過敏症症候群 - DIHSがつなぐ薬疹とウイルスとの関連性
(2011/11/11) - 線維芽細胞より作製したドパミン作動性ニューロンは生体内において機能的であるのか?
(2011/11/11) - 終末分化した肝細胞から機能的な神経細胞への直接的な系統転換
(2011/10/28) - Nerves and T Cells Connect
(2011/10/28) - Rapid and robust generation of functional oligodendrocyte progenitor cells
(2011/10/28) - 脂肪細胞が発毛を促進する!?
(2011/10/14) - ADAM13はClass B Ephrinsの分解とWntシグナルの調節により頭部神経冠を誘導する
(2011/10/14) - 多能性の維持に働くchromatin remodeling複合体esBAF
(2011/09/30) - 造血幹細胞の維持にはp57が重要である
(2011/09/30) - IGF-II : 記憶力がよくなる分子!?
(2011/09/16) - 固形腫瘍に存在する間葉系幹細胞は癌幹細胞を増加させる
(2011/09/16) - 小腸は抑制性Th17細胞の宝庫
(2011/09/02) - 細胞周期を制御する新規noncoding RNA
(2011/09/02) - Sema3A play an important role in remyelination failure in multiple sclerosis
(2011/08/19) - Drosophila Sex lethal Gene initiates Female Development in Germline Progenitors
(2011/08/19) - Wnt signaling is a key pathway for regulation of Melanocyte stem cells.
(2011/08/05) - A step closer to understanding the heart
(2011/08/05) - 神経再生を阻む「死」のシグナル
(2011/07/25) - テロメラーゼの再活性化によりマウスの組織老化が回復する
(2011/07/25) - 新遺伝子「Glis1」により、安全なiPS細胞を高効率に作製可能
(2011/07/08) - 幹細胞の"状態"をつくりだす細胞外環境
(2011/07/08) - 毛包幹細胞、色素幹細胞を維持
(2011/06/24) - BCL6を標的とした白血病の新たな治療戦略
(2011/06/24) - 自家移植におけるiPS細胞の免疫応答について
(2011/06/03) - ヒト疾患iPS細胞のウィルソン病への応用
(2011/06/03) - FOP(進行性骨化性線維異形成症)の異所性骨化部の起源は?
(2011/04/22) - 非対称分裂がNotchシグナルの活性化を介して皮膚の分化を促進する
(2011/04/22) - ショウジョウバエの腸管幹細胞の増殖は活性酸素により制御される
(2011/04/22) - 線維芽細胞からの直接的なエピブラストステムセルの誘導
(2011/04/08) - 抗リウマチ薬DHODH阻害剤はメラノーマの進展を抑える
(2011/04/08) - 癌再発の指標になる幹細胞
(2011/04/08)
- 新たに判明 がんの転移を促進するメカニズム
- 2010年度
ホーム > 世界の幹細胞(関連)論文紹介 > A step closer to understanding the ...
A step closer to understanding the heart
論文紹介著者

Daniela Yumi Kitashima
(博士課程 2年)
GCOE RA
Department of Dermatology
第一著者名・掲載雑誌・号・掲載年月
Jan Kajstura/Circ Res. 2010 Nov 26;107(11):1374-86. Epub 2010 Nov 18.
文献の英文表記:著者名・論文の表題・雑誌名・巻・号・ページ・発行年(西暦)
Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D'Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010 Nov 26;107(11):1374-86. Epub 2010 Nov 18.
論文解説
Cardiac regeneration has long been a dream of medicine. Heart disease leads the cause of death in developed countries and reach large proportion worldwide being a public health problem in many countries. The main mechanism of disease is cardiomyocytes*1 death, due to a limited capacity for muscle renewal upon injury. With scant capacity to regenerate dead cells, the heart cannot fulfill its function satisfactorily. Thus, rescuing cardiac muscle cell number and its function in the patients who are suffering of heart disease might help millions of people every year.
To achieve this ambitious goal, in recent years people have mainly concentrated on investigating adult and embryonic stem cells as well as potential source of exogenous stem cells and their influence on cardiac regeneration. Recent studies have documented that a tissue-specific stem cells resides in the human heart. It means that these stem cells can form new cardiomyocytes to replace old, dying cells within the myocardium in physiological conditions. It is extremely important to understand the mechanism involved in physiological turnover of cardiomyocytes and the origin of newly formed cells to apply this knowledge in so challenging cardiac regeneration.
Thus far, heart was considered an organ composed of a predetermined number of myocytes, which is established at birth and is preserved throughout life. Jan Kajstura et al. showed a novel conceptual framework of the heart: a self-renewing organ characterized by resident Human cardiac stem cells (hCSCs) stored in niches that controls the physiological turnover of cardiac cells. They studied the myocyte characteristics of 32 female and 42 male human hearts from patients 19 to 104 years of age who died from causes other than cardiovascular disease. They determined the level of p16INK4a protein that is associated with apoptosis*2. The rates of cell death found suggest a massive loss of myocytes with aging. If we consider the number of cells present in the left ventricle, the myocardium would diaper with time. In the absence of myocyte formation, only 5% of cardiomyocytes would persist at 63 and 48 years of age in women and men respectively. Thus, myocyte regeneration has to play a major role in the preservation of tissue mass and function of the aging human heart.
The possibility that a certain degree of myocyte regeneration occurs in the human heart is currently accepted, but questions persist concerning the origin of newly formed cardiomyocytes and the magnitude of myocyte regeneration. Kajstura's group study indicates that myocyte regeneration increase as a function of age as well as the pool of functionally competent Human cardiac stem cells (hCSCs) that generates a larger myocyte progeny. They showed that from 20 to 100 years of age, the yearly rate of myocyte turnover increases from 10% to 40% in female heart and from 7% to 32% in the male heart. From 19 to 104 years of age, essentially none of the myocytes present at bird is preserved in the young adult, middle-aged, and senescent hears. Furthermore, a mathematical analysis showed amazingly that the myocyte compartment is replaced 15 times in women and 11 times in men.
On the other hand, it is established that human aging is associated with a senescent cardiac phenotype defined by old cardiomyocytes, which have depressed mechanical performance. Thus, there is an apparent paradox between the increase in the number of senescent myocytes and the increase in myocyte renew with aging. If myocyte regeneration is increased with aging, it is expected that myocytes keep young. In an attempt to clarify this enigma, Kajstura's group measured telomere*3 length in hCSC and myocytes of 20 hearts. They found that old cardiac stem cells with severely shortened teomeres generate a myocyte progeny that rapidly acquires a senescent cell phenotype.
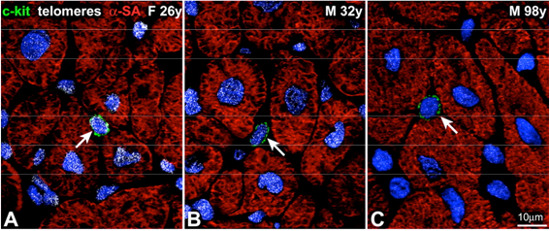
Organ aging and telomere length in hCSCs and myocytes. A through C, Detection of telomeres by Q-FISH (white dots) in c-kit-positive hCSCs (green arrows) and cardiomyocytes in the young female (A) and in the young (B) and old (C) male heart.
It means that a progressive loss of telomeric DNA in hCSCs occurs with aging and, although the pool of functionally competent hCSCs expands with the time and generates a larger myocyte progeny, the newly formed cardiomyocytes inherit short telomeres and rapidly reach the senescent cell phenotype. Aged hCSCs appear to generate old myocytes that may have severely depresses mechanical performance and alterations in calcium metabolism, contributing to the manifestations of aging myopathy in elderly.
In summary, this study brings us now closer to understanding human myocardial biology in terms of regeneration. The human heart is a highly dynamic organ regulated by a pool of resident hCSCs that modulate cardiac homeostasis and condition organ aging. If the human heart has innate regenerative response, it may be possible to exploit this therapeutically to enhance the heart's function upon injury. Thereby who knows, we are a step closer to open the door of the great dream of medicine: cardiac regeneration.
用語解説
- *1 muscle cells of heart
- *2 programmed cell death
- *3 region of repetitive DNA sequences at the end of a chromosome
Copyright © Keio University. All rights reserved.
