HOME > Program Members > Toshio Suda

Toshio Suda

Professor, Department of Cell Differentiation, Graduate School of Medicine, Keio University
Toshio Suda, MD, PhD
sudato@sc.itc.keio.ac.jp
http://www.coe-stemcell.keio.ac.jp/jp/member/suda.html
http://web.sc.itc.keio.ac.jp/celldiff/
Theme
The unique characteristics of stem cells, specifically their pluripotency and self-renewal ability, are critical for sustaining the lifelong functionality of organs. Stem cells reside in a special microenvironment called the niche. The stem cells interact with the niche via adhesion molecules and exchange molecular signals that allow maintenance of the specific features of these cells. For a better understanding of the nature of stem cells and their niches, we propose to characterize the proliferation and differentiation mechanisms of hematopoietic stem cells, germ stem cells and neural stem cells using cultures of sorted stem cells in vitro and a transplantation system in vivo. We have identified quite a few interesting molecules that are commonly expressed in different stem cells and that lead to unique phenomena depending on the local conditions. Through these experimental models and analyses, we aim to establish novel therapeutic approaches based on the interactions between the stem cells and their niches in the field of regenerative medicine.
Research activities
The quiescent state/slow cycling is thought to be a characteristic property for the maintenance of hematopoietic stem cells (HSCs). Interaction of HSCs with their particular microenvironments, known as the stem cell niches, is critical for adult hematopoiesis in the bone marrow (BM). We have demonstrated that quiescent HSCs adhere to osteoblasts in their niches through Tie2/ Angiopoietin-1 and/or mpl/thrombopoietin. When the BM is ablated during BM transplantation or after treatment with myelosuppressive agents, the quiescent HSCs enter the cell cycle and proliferate to supply progenitors of committed hematopoietic cells. We found that reactive oxygen species (ROS) induce the exit of HSCs from their niches after 5-FU injection, through downregulation of N-cadherin. Similarly, increase in ROS was observed after serial BM transplantation, and upregulation of p38MAPK and p16Ink4a was detected only in the HSCs.
Quiescent HSCs exist in the endosteal region of the bone marrow. When they begin to proliferate in response to various kinds of growth factors, they might move to the oxygen-rich environment near the vasculature. Thus, it is suggested that the proliferation of stem cells is blocked under the hypoxic condition. We are now studying the cellular metabolic changes of the HSCs, with the aim of showing that the metabolism of quiescent stem cells is glycolysis-dependent in hypoxic niches, using HIF-1α/VHL-deficient mice.
Moreover, we shall also discuss therapeutic strategies targeting the niches of cancer stem cells.
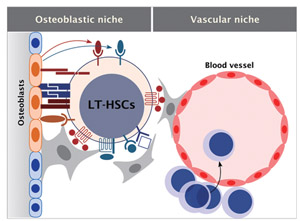
Fig.1 Hematopoietic stem cell niche
The niche for hematopoietic stem cells (HSCs)isthoughttoconsist concept-
Ually of two parts:the endosteal surface(osteoblasticniche) and a perivascular area(vascularniche). These two niches interact with each other and may play a complemental function for the maintenance of HSCs. Indeed, HSCs interacted with both osteoblasts and endothelial niche cells.
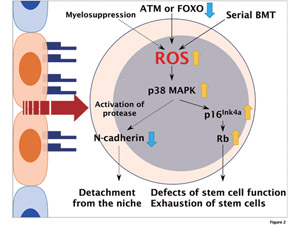
Fig.2
Role of reactive oxygen species (ROS) in the regulation of HSCs Elevation of ROS activates the p38 MAPK-p16Ink4a-retinoblastoma(Rb) pathway in HSCs, resulting in the defect of self-renewal activity and cell cycle quiescence. On the other hand, ROS induces the exit of HSCs from the niche after myelosuppression, through the downregulation of N-cadherin.
Selected Paper
- Arai, F., Hirao, A., Ohmura, M., Sato, H., Matsuoka, S., Takubo, K., Ito, K., Koh, G.Y., and Suda, T. (2004). Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149-161.
- Ito, K., Hirao, A., Arai, F., Matsuoka, S., Takubo, K., Hamaguchi, I., Nomiyama, K., Hosokawa, K., Sakurada, K., Nakagata, N., et al. (2004). Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997-1002.
- Ito, K., Hirao, A., Arai, F., Takubo, K., Matsuoka, S., Miyamoto, K., Ohmura, M., Naka, K., Hosokawa, K., Ikeda, Y., et al. (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12, 446-451.
- Takubo, K., Ohmura, M., Azuma, M., Nagamatsu, G., Yamada, W., Arai, F., Hirao, A., and Suda, T. (2008). Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell 2, 170-182.
- Yoshihara, H., Arai, F., Hosokawa, K., Hagiwara, T., Takubo, K., Nakamura, Y., Gomei, Y., Iwasaki, H., Matsuoka, S., Miyamoto, K., et al. (2007). Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1, 685-697.
Copyright © Keio University. All rights reserved.
