HOME > Program Members > Koichi Matsuo

Koichi Matsuo

Professor,Collaborative Research Resources,Graduate School of Medicine
Koichi Matsuo, MD, PhD
matsuo@sc.itc.keio.ac.jp
http://www.matsuo-lab.com
Theme
Bone is often viewed as the most promising target tissue in the field of regenerative medicine, and various strategies have been developed to differentiate bone-forming osteoblasts, both in vitro and in vivo. However, the molecular and cellular mechanisms of "bone remodeling" and "endochondral ossification (intracartilaginous ossification)" are not completely understood. Bone remodeling is a sequential process in which osteoclastic bone resorption is followed by osteoblastic bone formation, and this process is usually considered to maintain the bone size and shape. In contrast, endochondral ossification is a process to replace a cartilaginous template with bone. Endochondral ossification occurs both in the process of bone development and repair. All these processes are controlled by cell-cell interactions. In this project, we propose to analyze the interactions among bone cells, such as osteoclasts, osteoblasts, osteocytes and chondrocytes. Possible interactions with vascular endothelial cells and neurons will also be considered. First, we shall analyze roles of ephrinA ligands and EphA receptors in bone remodeling. By using bone fracture healing models in mice, we shall also analyze cross-talks between inflammatory cells and chondrocyte progenitors during endochondral ossification. How osteoblasts establish cell polarity to form bone during the process of endochondral ossification will be another focus of this project.
Research activities
Osteoclasts and osteoblasts communicate through cell-cell contact, diffusible paracrine factors and other modes (Fig. 1). Bone remodeling can be conveniently viewed as occurring in three phases: initiation, transition, and completion (1). We found that ephrinB2 expression is induced during osteoclast differentiation and that the reverse signaling through the ligand ephrinB2 in osteoclasts suppresses osteoclast differentiation, while forward signaling through the receptor EphB4 enhances osteoblast differentiation (2). Such ephrinB2-EphB4 interaction may facilitate the transition from bone resorption to bone formation during bone remodeling as one of the "coupling factors" (Fig. 2). We also analyzed bone remodeling in the auditory ossicles in the middle ear (3, 4).
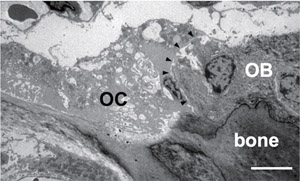
Fig.1 Osteoclast-osteoblas interaction. Transmission electronmicroscopic image of the mouse tibia shows an osteoclast(OC) in direct contact with an adjacent osteoblast(OB) on the bone surface.
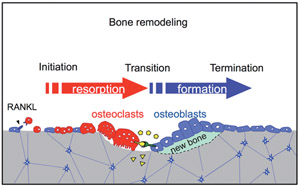
Fig.2 Bone remodeling and coupling factors. EphrinB2 and EphB4 are transmembrane proteins. Diffusible paracrine factors and molecules released from the bone matrix can also function as coupling factors(shown in yellow) balancing bone resorption and bone formation.
Selected Paper
- Matsuo K., Irie N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 473: 201-209, 2008.
- Zhao C., Irie N., Takada Y., Shimoda K., Miyamoto T., Nishiwaki T., Suda T., Matsuo, K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 4: 111-121, 2006.
- Kanzaki, S., Ito, M., Takada, Y., Ogawa, K., Matsuo, K. Resorption of auditory ossicles and hearing loss in mice lacking osteoprotegerin. Bone 39: 414-419, 2006.
- Kanzaki, S., Takada, Y., Ogawa, K., Matsuo, K. Bisphosphonate therapy ameliorates hearing loss in mice lacking osteoprotegerin. J. Bone Miner. Res. 24: 43-49, 2009.
- Matsuo, K. Cross-talk among bone cells. Curr. Opin. Nephrol.Hypertens. in press, 2009.
Copyright © Keio University. All rights reserved.
