HOME > Program Members > Shigeo Koyasu

Shigeo Koyasu

Professor, Department of Microbiology and Immunology, Graduate School of Medicine, Keio University
Shigeo Koyasu, D.Sc.
koyasu@sc.itc.keio.ac.jp
http://www.koyasu.umin.ne.jp
Theme
Isolation and transplantation of tissue-specific stem cells
Regulation of inflammatory responses based on an understanding of the onset of immune reactions governed by dendritic cells" (Koyasu laboratory)
The goal of this research group is to control inflammatory responses based on understanding of the system of immune reactions governed by dendritic cells (DCs). It is critical for the homeostasis of the immune system that appropriate immune responses are induced against antigens, including foreign as well as self-antigens. Hyper-immune responses would result in the onset of allergic responses, inflammatory diseases and autoimmune diseases, while deficient responses may cause infectious diseases. DCs present antigens to T lymphocytes and induce the differentiation of naive T cells into Th1, Th2 and Th17 cells, which play central roles in different types of inflammatory responses. Regulatory T cells, on the other hand, suppress immune reactions for overall homeostasis of the immune responses. We propose to study the roles of the DCs in both innate and adaptive immune responses by focusing on the regulation of cytokine gene expression by these cells and elucidate a novel control method(s) to regulate the inflammatory responses involved in the infection, wound healing and tissue regeneration.
Research activities
- We demonstrated that the IL-15/IL-15 receptor system plays an important role in the functional maturation of dendritic cells (DCs) by controlling the cytokine cascade. We also showed that DC-derived IL-15 is critical for the induction of inflammatory responses to bacterial infections.
- IL-12 produced by DCs is a critical cytokine for inducing a Th1 response. We present evidence indicating that the phosphoinositide 3-kinase (PI3K) negatively regulates the IL-12 expression in DCs. We further examined the downstream signaling pathways, and demonstrated, both in vivo and in vitro, that mTOR and GSK3β negatively and positively regulate IL-12 expression, respectively. We further showed that the inhibition of mTOR and GSK3β augment and reduce Th1 responses, respectively.
- We showed that the redox state and oxygen concentration affect the cytokine expressions in DCs, and that DCs produce less IL-12 and more IL-10 under the hypoxic condition as compared to the normoxic condition.
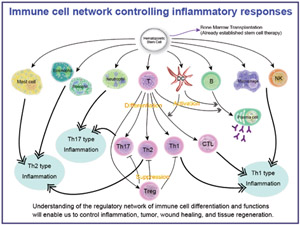
Fig.1
Differentiation of immune cells involved in various inflammatory responses: immune cells originate from haematopoietic stem cells. It is important to understand the regulatory network of immune cell differentiation and functions in order to control inflammation, tumor, wound healing and tissue regeneration.
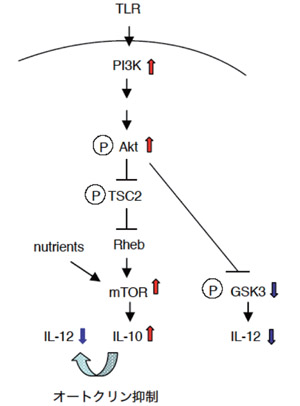
Fig.2
Regulatory mechanisms by PI3K to control IL-12 production from DCs (Ohtani et al. 2008. Blood 112:635-643.)
Selected Paper
- Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S. and Koyasu S. mTOR and GSK3 differentially regulate LPS-induced IL-12 production in dendritic cells. Blood 112:635-643 (2008).
- Ohteki T, Tada H, Ishida K, Sato T, Maki C, Yamada T, Hamuro J. and Koyasu S. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J Exp Med. 203:2329-2338 (2006).
- Suzuki H, Matsuda S, Terauchi Y, Fujiwara M, Ohteki T, Asano T, Behrens TW, Kouro T, Takatsu K, Kadowaki T. and Koyasu S. PI3K and Btk differentially regulate B cell antigen receptor mediated signal transduction. Nat Immunol. 4:280-286 (2003).
- Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T. and Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in dendritic cells. Nat Immunol.3:875-881 (2002).
- Ohteki T, Suzue K, Maki C, Ota T, and Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol. 2:1138-1143 (2001).
Copyright © Keio University. All rights reserved.
